Medical devices - Quality management systems - Requirements for regulatory purposes
Available for SubscriptionsBS EN ISO pdf download BS EN ISO,Medical Devices—Quality Management Systems—Requirements For Regulatory Purposes (British Standard) BS EN ISO PDF can be downloaded free of charge on this website.BS EN ISO replaces BS EN ISO. ISO specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. ISO 13485 Third edition 2016-03-01 Reference number ISO (E) Licensed to Red Star Contract Mfg / Barry Leffers (barry@redstarcontractmfg.com) ISO Store Order: OP-125087 / Downloaded: 2016-02-29 Single user licence only, copying and networking prohibited. ISO specifies more detail than 21 CFR 820 and addresses competence as opposed to training e g competence via education skills experience 21 CFR 820 specifies requirements for 1 personnel performing verification and validation activities and 2 21 CFR 820 states that personnel shall be made aware 26-Nov-20200 Views15 Pages.
Available in Packages- ISO 13485 / 14971 / 14969 - Medical Devices Package
- ISO 13485 / IEC 62304 / ISO 14971 - Medical Devices Package
- ISO 13485 and ISO 14971 - Medical Devices Package
- ISO 13485 / ISO 9001 - Medical Devices Quality Management Set
- ISO 13485 and ISO/TR 14969 Quality Management Systems Medical Devices Package
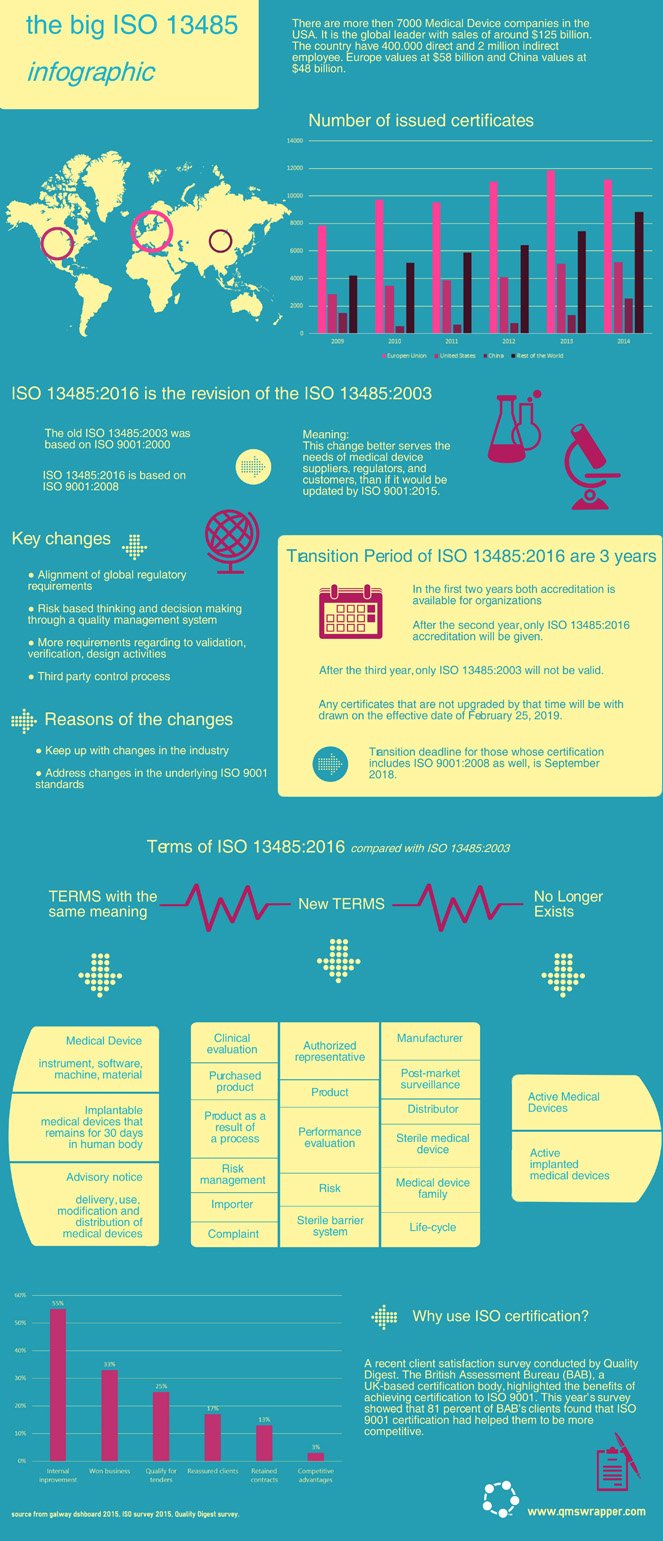
- ISO 13485:2016 and ISO 13485:2003 - Medical Devices Transition Set
- ISO 9001 / ISO 13485 - Quality Management for Medical Devices Set
Content Provider
International Organization for Standardization [ISO]

Your Alert Profile lists the documents that will be monitored. If the document is revised or amended, you will be notified by email. You may delete a document from your Alert Profile at any time. To add a document to your Profile Alert, search for the document and click 'alert me'.
Please first verify your email before subscribing to alerts.Your Alert Profile lists the documents that will be monitored. If the document is revised or amended, you will be notified by email. You may delete a document from your Alert Profile at any time. Download game kingdom and lords java. To add a document to your Profile Alert, search for the document and click 'alert me'.
Already Subscribed to this document.Your Alert Profile lists the documents that will be monitored. If the document is revised or amended, you will be notified by email. You may delete a document from your Alert Profile at any time. To add a document to your Profile Alert, search for the document and click 'alert me'.

BSI's 'ISO 13485:2016 Lead Auditor' competency-based 4-day course teaches a general understanding of the concepts of the ISO 13485:2016 standard and the principles and practices of leading management systems and process audits in accordance with ISO 19011:2018, 'Guidelines on Auditing Management Systems'. Experienced instructors explain the clauses of ISO 13485:2016 in detail and guide students through the entire audit process, from managing an audit program to reporting on audit results. Our qualified instructors will also help you to boost your audit capabilities with the latest developments of the new 19011 standard. Students gain necessary auditing skills through a balance of formal classroom tutorials, role playing, group workshops, and open forum discussions.
This course comprises the following three Exemplar Global TPECS Competency Units whose outcomes are certified by Exemplar Global:
- MD - Medical Device Systems
- AU - Management Systems Auditing
- TL - Leading Management Systems Audit Teams
Attendees successfully completing this course receive a Certificate of Attainment for each of Exemplar Global Competency Units listed above.
New to TPECS?
For more information, please see our Understanding the TPECS Course Structure page.
Learning Objectives
- Understand quality management definitions, concepts, and guidelines
- Understand the purpose of the ISO 9000 series
- Understand the requirements of the ISO 13485:2016 standard
- Understand the roles and responsibilities of the auditor
- Apply ISO 19011:2018 concepts, new terminology and guidelines
- Understand the types of risks and opportunities associated with auditing
- Recognize the principles, practices, and types of audits
- Conduct all phases of an audit adopting a risk-based approach, particularly in the section of audit planning
- Prepare and present effective reports
- Understand Exemplar Global's certification scheme
- Understand the role of objectives, scope and criteria in the audit process
- How to plan audits
- Conduct audit team selection
- Initiate the audit and conducting opening meetings
- Understand audit team leader responsibilities
- Communicate effectively during the audit
- Apply the latest auditor techniques and identify appropriate use
- Conduct on-site activities
- Prepare audit conclusions
- Conduct closing meetings
- Report audit results
Course Materials
You will have the option to receive your course materials in the following formats:
- Hard Copies: At the start of the course, a physical copy of the Student Handbook will be provided.
- Soft Copies: Prior to the course, an email with instructions on how to access the online Student Handbook, which can be viewed, downloaded or printed, will be sent to students.
.png)
NOTE: Copies of the standards are not included in the class fee. BSI will make reasonable efforts to have loaner copies available for use during the class, but students are encouraged to bring their own copy. Soft copies of the digital loaner standard cannot be printed or downloaded.

Your Alert Profile lists the documents that will be monitored. If the document is revised or amended, you will be notified by email. You may delete a document from your Alert Profile at any time. To add a document to your Profile Alert, search for the document and click 'alert me'.
Please first verify your email before subscribing to alerts.Your Alert Profile lists the documents that will be monitored. If the document is revised or amended, you will be notified by email. You may delete a document from your Alert Profile at any time. Download game kingdom and lords java. To add a document to your Profile Alert, search for the document and click 'alert me'.
Already Subscribed to this document.Your Alert Profile lists the documents that will be monitored. If the document is revised or amended, you will be notified by email. You may delete a document from your Alert Profile at any time. To add a document to your Profile Alert, search for the document and click 'alert me'.
BSI's 'ISO 13485:2016 Lead Auditor' competency-based 4-day course teaches a general understanding of the concepts of the ISO 13485:2016 standard and the principles and practices of leading management systems and process audits in accordance with ISO 19011:2018, 'Guidelines on Auditing Management Systems'. Experienced instructors explain the clauses of ISO 13485:2016 in detail and guide students through the entire audit process, from managing an audit program to reporting on audit results. Our qualified instructors will also help you to boost your audit capabilities with the latest developments of the new 19011 standard. Students gain necessary auditing skills through a balance of formal classroom tutorials, role playing, group workshops, and open forum discussions.
This course comprises the following three Exemplar Global TPECS Competency Units whose outcomes are certified by Exemplar Global:
- MD - Medical Device Systems
- AU - Management Systems Auditing
- TL - Leading Management Systems Audit Teams
Attendees successfully completing this course receive a Certificate of Attainment for each of Exemplar Global Competency Units listed above.
New to TPECS?
For more information, please see our Understanding the TPECS Course Structure page.
Learning Objectives
- Understand quality management definitions, concepts, and guidelines
- Understand the purpose of the ISO 9000 series
- Understand the requirements of the ISO 13485:2016 standard
- Understand the roles and responsibilities of the auditor
- Apply ISO 19011:2018 concepts, new terminology and guidelines
- Understand the types of risks and opportunities associated with auditing
- Recognize the principles, practices, and types of audits
- Conduct all phases of an audit adopting a risk-based approach, particularly in the section of audit planning
- Prepare and present effective reports
- Understand Exemplar Global's certification scheme
- Understand the role of objectives, scope and criteria in the audit process
- How to plan audits
- Conduct audit team selection
- Initiate the audit and conducting opening meetings
- Understand audit team leader responsibilities
- Communicate effectively during the audit
- Apply the latest auditor techniques and identify appropriate use
- Conduct on-site activities
- Prepare audit conclusions
- Conduct closing meetings
- Report audit results
Course Materials
You will have the option to receive your course materials in the following formats:
- Hard Copies: At the start of the course, a physical copy of the Student Handbook will be provided.
- Soft Copies: Prior to the course, an email with instructions on how to access the online Student Handbook, which can be viewed, downloaded or printed, will be sent to students.
NOTE: Copies of the standards are not included in the class fee. BSI will make reasonable efforts to have loaner copies available for use during the class, but students are encouraged to bring their own copy. Soft copies of the digital loaner standard cannot be printed or downloaded.
Who Should Attend?
- Individuals interested in conducting first-party, second-party, and third-party audits
- Management Representatives
- Quality Directors in Medical Device
- Managers
- Engineers
- Consultants
Iso 13485 Pdf Free Download
Prerequisite
A prior review of the ISO 13485:2016 standard is required and internal audit experience is suggested for this course.
Class Logistics
Iso 13485 2016 Pdf Deutsch
There are written tests on each of the competency units in turn on Days 2, 3 and 4. Detailed exam instructions will be provided. Certificates of Attainment in each competency unit will be provided for students who are deemed 'Competent' for each competency unit. Certificates of attendance are provided to those who do not pass the competency test(s), and students will be given the opportunity to retake the test(s).
Online course option
Iso 13485 2016 Changes
This Course is also available as part of BSI's Connected Learning Live service. A virtual instructor lead training course taught live online in a virtual classroom. Scheduled times and dates are available HERE
